age-associated thymus dysfunction
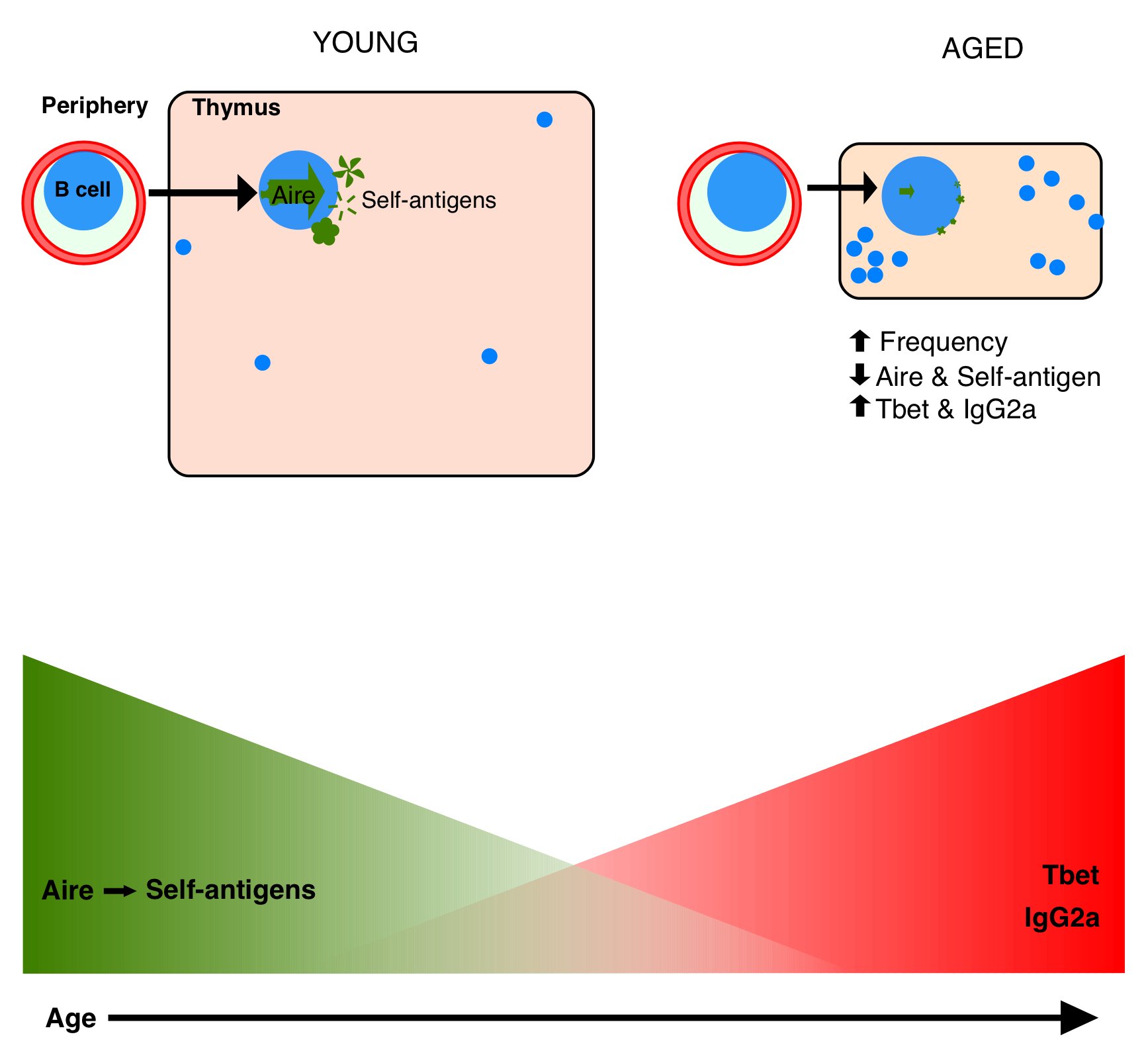
The thymus begins to atrophy relatively early in life, causing a decline in generation of new, naive T cells. This is compensated by homeostatic expansion of memory T cells in the periphery, which restricts the T cell receptor repertoire, leaving older people less responsive to vaccines, more susceptible to new infections, and with diminished tumor surveillance. Thymic stromal cells are the primary targets of age-associated atrophy, and acquire high levels of oxidative damage due to low catalase expression. In addition to loss of individual stromal cell size, this also diminishes the capacity of stromal cells to enforce self-tolerance in the developing T cell population, and contributes to age-associated increases in susceptibility to autoimmune disease. We are investigating the mechanisms that regulate diminished central T cell tolerance induction, including age-associated dysfunction of thymic B cells’ expression of the autoimmune regulator Aire.
Paper links: Cepeda 2016 (review), Cepeda 2018, Hester 2022
Current funding: R21AI154109
Past funding: R01AI121367